FDA 510(k) Clearance Granted for Endomag’s Breast Tumor Localization System
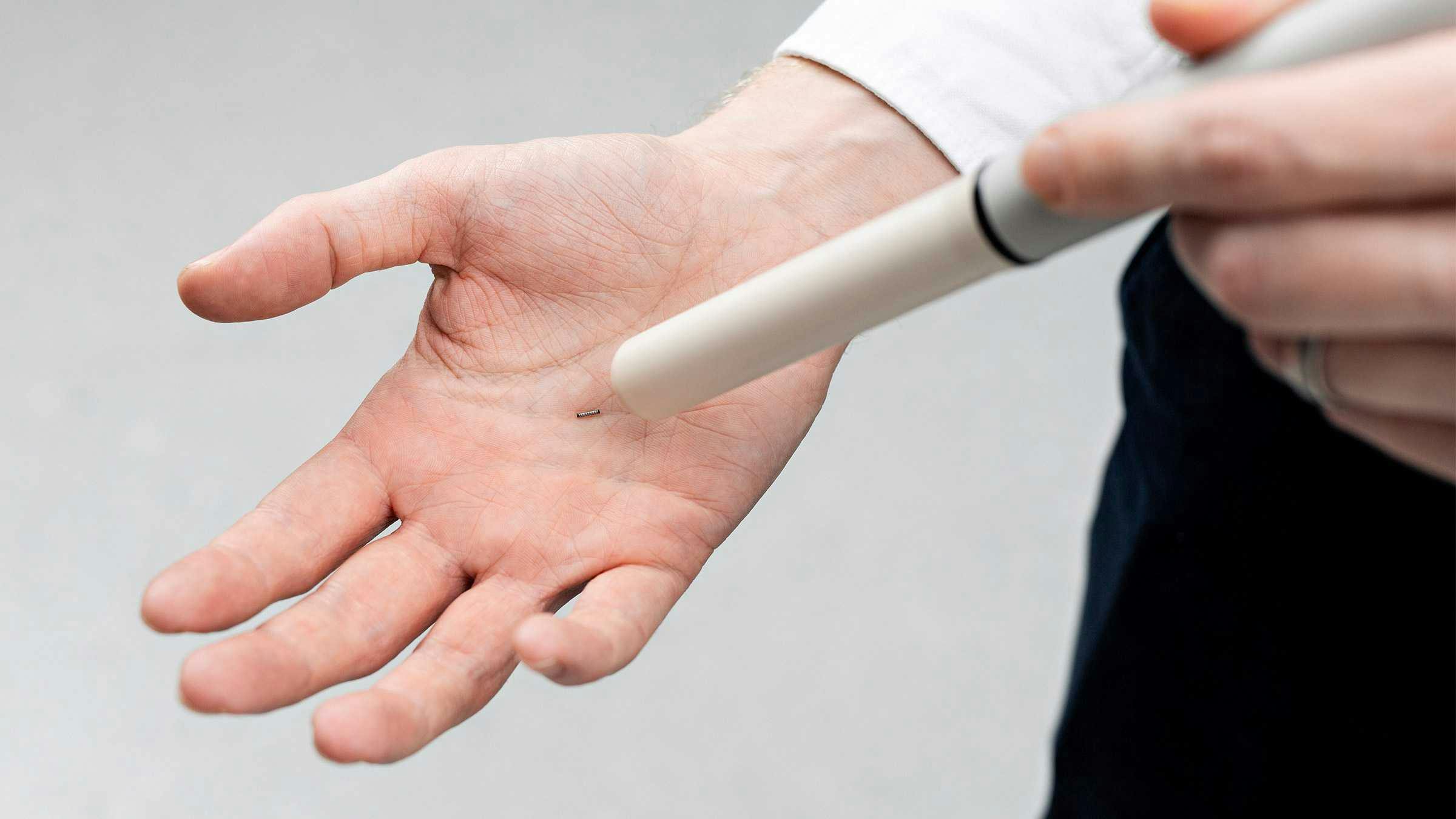
Endomag has announced that it has received 510(k) clearance from the U.S. Food and Drug Administration (FDA) for its Magseed® magnetic marker for use with Sentimag®.
Expanding its solutions for cancer clinicians and patients, Endomag’s Magseed® is designed to guide surgeons using Sentimag® during a breast lumpectomy.
Magseed® can be placed by radiologists up to 30 days in advance of surgery using ultrasound or radiographic guidance, offering scheduling flexibility for surgeons and radiologists compared to wire-guided localization.
Traditional guide wires protrude from a patient’s breast, leading to potential discomfort and restricting the patient to the hospital before surgery. More critically, the protruding guide wires risk movement prior to surgery, requiring follow-up surgery in up to 55% of lumpectomy procedures.
Magseed® is designed for patient flexibility too, as it is similar in size to a grain of rice and placed within the tumor, allowing the possibility for patients to return home before surgery.
As breast cancer screening programs have advanced, tumors are now detected much earlier when they are still small. Regular mammograms remain the best way for clinicians to find breast cancer early, sometimes up to three years before a tumor can be felt. As these small tumors are often impalpable, they are difficult for surgeons to locate during a lumpectomy.
Wire-guided localization was introduced during the 1970’s whereby a radiologist inserts a guide wire that the surgeon follows to the tumor via dissection. While effective enough to become the standard of care, clinicians have consistently searched for better options.
"We are extremely pleased to receive this clearance and to have the opportunity to support U.S. clinicians and patients with our products"Dr Eric Mayes CEO, Endomag
The Magseed® is located using audio-visual cues from the Sentimag®, a magnetic surgical guidance device that received its CE mark approval in 2010 and has been used in over 12,000 breast cancer procedures across Europe with Endomag’s third product, Sienna+®.
Endomag will announce U.S. availability of Magseed® and Sentimag® during Q2’16 and will meet with surgeons and radiologists at the upcoming SBI/ACR Breast Imaging Symposium in Austin, Texas on April 7-10 and the American Society of Breast Surgeons (ASBrS) in Dallas, Texas on April 13-17.
Dr Eric Mayes, CEO at Endomag, said:
“We are extremely pleased to receive this clearance and to have the opportunity to support U.S. clinicians and patients with our products. In addition, we are excited to be launching our third product since we entered the European market in 2013. This clearance highlights our ability to deliver an exciting pipeline of innovative products to a global market.”
Mayes noted that Magseed® availability is expected during Summer 2016 for new customers in Europe, Middle East and Africa (EMEA) together with those currently using Sentimag® with Sienna+® for lymph node localization. Other active markets will follow.