European breast cancer patients can now access more convenient and targeted treatment with new Magseed® marker indication
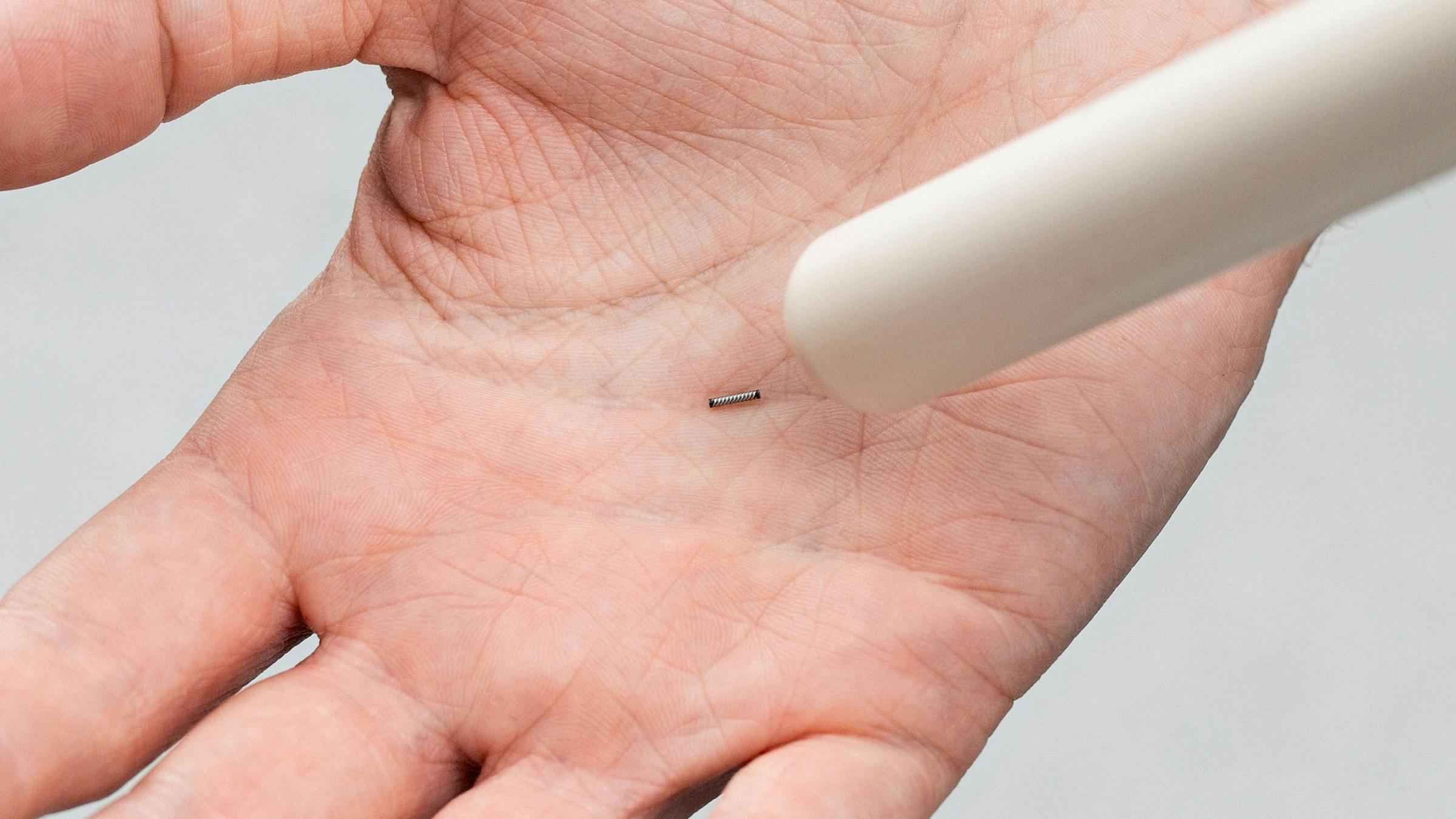
Magseed® magnetic marker approved in Europe for long-term implantation in any soft tissue.
Endomag, the market leader for seed localisation in Europe, has received an extended indication for the long-term use of the Magseed® marker in any soft tissue.
The Magseed® marker has already enabled tens of thousands of women to benefit from the more accurate marking and removal of breast tumours.
This new indication makes the Magseed® marker the first localisation seed in Europe that physicians can place days, weeks or even months ahead of surgery to mark any cancer site for accurate removal.
The Magseed® marker has quickly established itself as the market leader in Europe for seed localisation. The tiny seed, made from surgical grade steel, is placed in tissue to mark tumours before surgery to help the surgeon accurately locate the cancer in the operating room and remove it in one piece.
Once placed, it cannot be dislodged or damaged and is detected by the Sentimag® probe with millimetre precision.
Dr. James Harvey discusses why being able to mark lymph nodes is beneficial for patients
Many thousands of patients to benefit from new techniques now possible
Dr. James Harvey, Consultant Oncoplastic Breast Surgeon, Manchester University NHS Foundation Trust said:
“When it comes to marking lymph nodes in patients receiving neo-adjuvant chemotherapy there are very few options – wires are notoriously difficult to place and can easily migrate, often resulting in multiple lymph nodes being removed but not the one you necessarily wanted to take.
It’s a huge step forward that we can now use the Magseed® marker to mark lymph nodes across Europe, which will allow us to offer our patients more targeted surgery and accurate staging.”
Eric Mayes, CEO at Endomag said:
“Ever since we launched the Magseed® marker we have been asked by European physicians if they can use it to mark a variety of different cancer sites in the body, which also require a longer implantation than the 30 days we had in Europe. We received this expanded indication from the FDA in the US last year and I’m delighted that European cancer patients can now access this same level of targeted treatment.”
Dr. Abigail Caudle (MD Anderson) and Dr. Michael Alvarado (University of California, San Francisco) on the benefits of targeted axillary surgery
Dr. Abigail Caudle, Associate Professor, Department of Breast Surgical Oncology from the University of Texas MD Anderson Cancer Center comments:
“The ability to mark lymph nodes and ensure we remove the cancerous node to examine them closely is a huge advantage for patients, because we spare them extensive and unnecessary axillary surgery”
Cancers can now be marked not just before surgery, but before chemotherapy too
Thanks to the rapid progression in breast cancer treatment, more women are receiving chemotherapy before surgery to help shrink their tumour.
However, treating the tumour so early makes it difficult to determine if the cancer has spread beyond the breast when the patient comes to surgery.
With this new indication, the Magseed® marker can now be used to mark suspicious lymph nodes before chemotherapy, and in conjunction with Endomag’s lymphatic mapping agent, Magtrace®, will allow the surgeon to perform a more targeted dissection to increase the accuracy of the surgery and determine if the cancer has spread.
Results from two independent studies conducted by Dr Abigail Caudle from MD Anderson Cancer Center and Dr Heather Greenwood, University of California, San Francisco, demonstrated the ease and accuracy of marking positive lymph nodes with a Magseed® marker and 100% retrieval success.
Extensive clinical evidence was demonstrated to support the expanded indication
The extended indication was approved after the Magseed® marker was found to be accurate and easy to place in the breast and the armpit in a number of clinical studies.
In data presented from MD Anderson Cancer Center, Houston, University Hospitals, Cleveland and University of California, San Francisco, it was demonstrated that the Magseed® marker is easy to place in the tumour, does not move once in place and has a very low rate of patients needing a second surgery to remove residual disease, e.g. MDA 6.5%, UCSF 7.5%, UH 10%.
In the United States the Magseed® marker received 510(k) clearance from the U.S. Food and Drug Administration (FDA) for the same indication in 2018.